
The EU’s ‘Digital Green Certificate’ aims to ease travel within the bloc this summer (Photo: rudlavibizon)
EU commissioner for justice Didier Reynders on Tuesday (13 April) announced that a pilot project for EU-wide vaccine certificates could be launched “in the beginning of June” – aiming to have the whole system operating by the end of June.
But both MEPs and member states still have to give green light to the proposal.
All EU-approved vaccines will be accepted for the document. Individual member states can decide to accept other vaccines, such as the Russian or Chinese ones (Photo: European Commission)
Only 13 member states have agreed on the specific criteria for issuing such a green passport for tourism by this June “at the latest,” according to an announcement by the Austrian tourism ministry on Monday.
These are Austria, Bulgaria, Croatia, Cyprus, Denmark, France, Germany, Greece, Italy, Malta, Portugal, Slovenia, and Spain.
Member states will be able to extend the use of these certificates beyond tourism.
For their part, most MEPs have been vocal in support for the proposal put forward by the EU Commission, but key questions remain – ranging from fundamental rights to scientific validity.
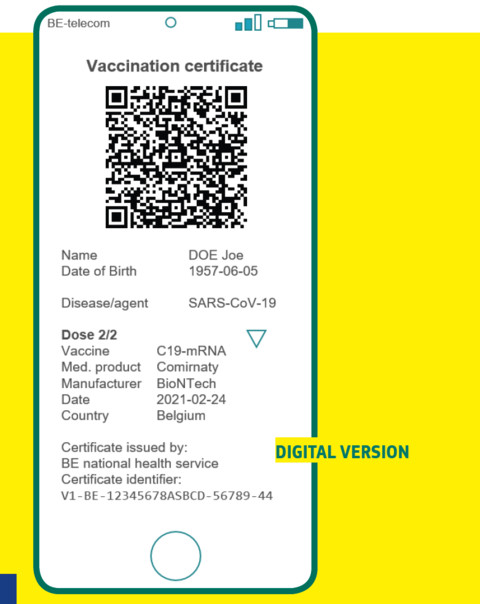
The so-called Digital Green Certificate will provide proof of COVID-19 vaccination, recovery, or test results (both PCR test or rapid antigen test), with a QR code to ensure security. It will be free and available in digital form or on paper.
The EU Commission insists that this tool will prevent discrimination against individuals who are not vaccinated, but some MEPs remain skeptical about how the certificates will work in practice.
Under the commission proposal, EU countries have to issue vaccination certificates for people who have received any of the jabs approved by the European Medicine Agency. They can decide to accept other vaccines that have only been approved in a specific member state.
So far, BioNTech/Pfizer, Moderna, AstraZeneca, and Johnson&Johnson vaccines are the only jabs approved in the EU.
But Hungary, for example, has also approved the Russian Sputnik V vaccine and the Chinese Sinopharm jab.
As a result, EU citizens vaccinated with non-EMA-approved jabs might find more obstacles to travel within the bloc than Chinese and Russian tourists, Liberal MEP Yana Toom warned.
Affordable, or free, tests?
While the EU executive has repeatedly stressed that these certificates cannot be used as “a pre-condition to free movement” in the EU, fellow liberal MEP Sophie in ‘t Veld warned that they will be in practice.
“If you cannot show a certificate then you will be stopped at the border. It is that simple, so let’s be honest about it,” she added, pointing out that tests need to be affordable in all member states to be a true alternative for vaccination.
This idea is also supported by Green MEPs, who announced an amendment to the commission’s proposal, stressing that testing should be free for all.
In the EU, average costs differ significantly among member states.
In the Netherlands or Ireland, for example, the price of a travel-related PCR is between €100 to €160, while in Belgium it is about €50.
Yet, commissioner Reynders said that “making these test free of charge or capping their price would constitute a serious interference of member states competence in the field of public health”.
Meanwhile, the fact that the measure will be suspended only once the World Health Organization declares the end of the Covid-19 pandemic has also triggered doubts among EU lawmakers.
According to MEP Nicola Procaccini, from the European Conservatives and Reformists group, “a European instrument should not be in the hands of a non-EU organization”.
False expectations?
Another concern looms: the ‘recovery’ part of the certificate.
The European Data Protection Supervisor, Wojciech Wiewiórowski, said that there is little scientific evidence that the recovery from the disease grants any kind of immunity.
“Even if from a data protection point of view system is safe, its operation should not lead to the creation of false expectations,” he said.
“We cannot spread fake safety assurances which are not founded on solid scientifically proven knowledge,” he added.
The certificate proposal will not require any centralized database at the EU level.
The EU parliament aims at adopting its mandate for starting negotiations with the EU Council in the next plenary session (26-29 April).
Source: https://euobserver.com/coronavirus/151529
[Disclaimer]








